Summary
Treatment with bendavia in patients receiving percutaneous coronary intervention for STEMI in the EMBRACE STEMI trial did not result in a reduction in reperfusion injury-related outcomes compared with placebo. However, these data have led to further hypotheses, which are currently under investigation in clinical trials.
- EMBRACE
- STEMI
- bendavia
- reperfusion injury
- percutaneous coronary intervention
- stenting
- cardiology & cardiovascular medicine clinical trials
- NCT01572909
C. Michael Gibson, MD, MS, Harvard Medical School, Boston, Massachusetts, USA, presented results from the EMBRACE trial [NCT01572909], which investigated the effect of bendavia administration on reperfusion injury in patients receiving percutaneous coronary intervention (PCI) and stenting for STEMI. EMBRACE was an international, multicenter trial designed to investigate the effect of bendavia on infarct size in patients with first-time anterior STEMI. Patients were required to present with a closed artery and a TIMI flow of 0 or 1 in proximal or mid left anterior descending lesions, and within 4 hours of symptoms. Patients with shock were excluded. The primary analysis population was smaller than planned due to low numbers of patients presenting with complete obstruction on angiography.
Mitochondria present a potential target for pharmacotherapy to lower reperfusion injury. In animal studies, bendavia, a mitochondria-targeting peptide, has reduced infarct size following ischemic events up to 42% [Kloner RA et al. J Am Heart Assoc. 2012], and improvements occur with no changes in heart rate or blood pressure [Sabbah HN et al. Eur Heart J. 2013 (abstr P3286)]. These results showed promise of improved mitochondrial bioenergetics without increased demands on the heart.
Patients were randomized 1:1 and were blinded to treatment with bendavia (n = 150) or volume-matched placebo (n = 147). Bendavia IV was administered at 0.05 mg/kg/h at least 15 minutes prior to, and 60 minutes following, PCI. The primary end point was the area under the curve (AUC) for serum creatine kinase MB (CK-MB) levels over 72 hours post-PCI. The clinical end point was a composite of all-cause death, new-onset congestive heart failure (CHF), and CHF rehospitalization. Secondary end points included infarct size by the AUC for troponin I, magnetic resonance imaging outcomes, TIMI perfusion grade and corrected TIMI frame count post-PCI, and ST-elevation resolution.
Of 297 randomized patients, an unexpected 117 (40%) had an open artery at angiography (pre-PCI TIMI flow grade > 1) and were excluded from the analysis. After satisfying all patient exclusions, the primary analysis population was 118; patients were excluded from the treatment arms equally. Baseline characteristics were similar except hypertension (60% in placebo group vs 37.9% in treatment group). Other clinical and angiographic characteristics were similar.
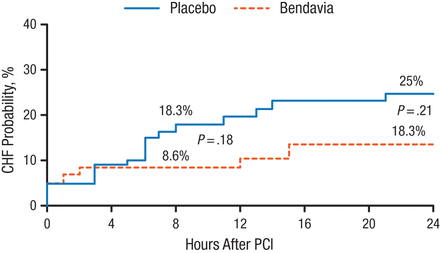
The primary end point in the study showed no significant difference between the study drug group and the placebo group. No differences were seen in the clinical composite end point or any secondary end points. The exploratory end point of CHF was numerically lower with bendavia at 8 hours following treatment; however, this difference was not statistically significant (Figure 1).
CHF Probability for 24 Hours Following PCI
CHF, congestive heart failure; PCI, percutaneous coronary intervention.
Reproduced with permission from CM Gibson, MS, MD.
Non-prespecified, exploratory analyses found potential benefit in patients with hypertension who received bendavia for infarct volume and ST-segment resolution, and a trend toward benefit for edema volume. Exploratory analyses showed several protective renal outcomes associated with bendavia.
Dr Gibson concluded by reiterating that no differences were seen between bendavia and placebo in terms of the CK-MB AUC, but added that these data generated hypotheses toward the potential for bendavia to reduce CHF symptoms within 8 hours following PCI, which is under investigation for patients with systolic heart failure in additional clinical trials. The potential renal protective effects of bendavia are also under investigation.
- © 2015 SAGE Publications