Summary
This article presents data from A Safety and Efficacy Study of Oral MDV 3100 [enzalutamide] in Chemotherapy-Naïve Patients With Progressive Metastatic Prostate Cancer [PREVAIL; NCT01212991; Beer TM et al. N Engl J Med 2014]. Enzalutamide, an androgen receptor inhibitor, has been shown to improve overall survival (OS) and radiographic progression-free survival (rPFS) in men with metastatic castrate-resistant prostate cancer (mCRPC) who had received docetaxel therapy [Scher H et al. N Engl J Med 2012]. PREVAIL researchers examined whether enzalutamide could prolong OS and rPFS in men with mCRPC who had progressed on androgen deprivation therapy.
- Oncology Clinical Trials
- Reproductive Cancers
- Oncology Clinical Trials
- Oncology
- Reproductive Cancers
Andrew Armstrong, MD, Duke Department of Medicine, Durham, North Carolina, USA, provided data from A Safety and Efficacy Study of Oral MDV 3100 [enzalutamide] in Chemotherapy-Naïve Patients With Progressive Metastatic Prostate Cancer [PREVAIL; NCT01212991; Beer TM et al. N Engl J Med 2014]. Enzalutamide, an androgen receptor inhibitor, has been shown to improve overall survival (OS) and radiographic progression-free survival (rPFS) in men with metastatic castrate-resistant prostate cancer (mCRPC) who had received docetaxel therapy [Scher H et al. N Engl J Med 2012]. PREVAIL researchers examined whether enzalutamide could prolong OS and rPFS in men with mCRPC who had progressed on androgen deprivation therapy (ADT).
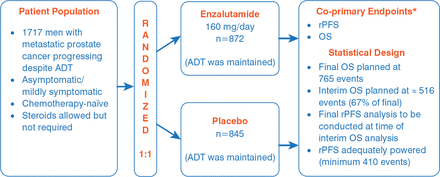
The multinational PREVAIL—a randomized, double-blind, placebo-controlled study—enrolled asymptomatic or mildly symptomatic men to enzalutamide (160 mg/day; n=872) or placebo (n=845), with respective median treatment durations of 16.6 and 4.6 months. The coprimary endpoints were OS and rPFS, and these were analyzed for the intent-to-treat population (Figure 1). Other efficacy endpoints included time to cytotoxic chemotherapy, time to neoplastic treatment, time to first skeletal-related event, time to prostate-specific antigen (PSA) progression, time to Functional Assessment of Cancer Therapy-Prostate degradation, best objective response, and PSA decline from baseline. The treatment groups were well balanced relative to baseline patient characteristics and baseline disease burden.
PREVAIL Trial Design
ADT=androgen deprivation therapy; OS=overall survival; rPFS=radiographic progression-free survival.
*Total alpha=0.05, 2-sided (OS allocation=0.049; rPFS allocation=0.001); target hazard ratio: OS=0.82, rPFS=0.57.
Reproduced with permission from A Armstrong, MD.
Enzalutamide, when compared with placebo, significantly prolonged rPFS (65% vs 14% at 12 months; p<0.0001), reduced the risk of death by 29% (p<0.0001), delayed the median time to chemotherapy by 17 months (HR, 0.72), and delayed time to PSA progression (p<0.0001). With enzalutamide, there was an objective soft tissue response (complete response plus partial response) of 59%, compared with 4.9% for placebo (p<0.0001), and a reduced time until the first skeletal-related event (HR, 0.72; p<0.001). Furthermore, enzalutamide was beneficial over placebo for the rate of decline of ≥50% in PSA (78% vs 3%; p<0.001). Dr. Armstrong also provided an updated OS analysis (Table 1).
Updated OS Analysis as of January 15, 2014a
Given a planned interim analysis at 540 deaths, the Data Monitoring Committee recommended that the study be stopped and that patients in the placebo group cross over to the enzalutamide group. The most common adverse events with a higher incidence in the enzalutamide arm than the placebo arm were fatigue (35.6% vs 25.8%), back pain (27.0% vs 22.2%), constipation (22.2% vs 17.2%), and arthralgia (20.3% vs 16.0%). Seizure was reported in 1 patient in each treatment arm (0.1%).
In summary, for men with mCRPC who have progressed on ADT but have not received chemotherapy, treatment with enzalutamide has a favorable safety profile and significantly improves OS, rPFS, and secondary measures of disease response and progression.
- © 2014 MD Conference Express®